

Diatoms are unicellular, photoautotrophic algae which surround themselves with two halves of a silica shell, the epi - and the hypotheca. These shells consist of an organic/inorganic composite material. Amorphous SiO2 is interspersed with organic, long chain polyamines or silaffins. Both components promote the biomineralization of soluble [Si(OH)4] to SiO2 also in vitro.
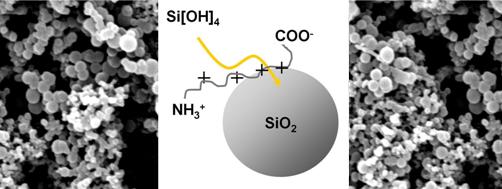
Silaffins are short peptides of 4 -8 kDa mass which carry massive posttranslational modifications. Besides the more common phosphorylation of serin residues they are characterized by a unique pattern of lysin modifications which consist of methylations, hydroxylations and attached propyleneimine moieties. These modified serin and lysin residues confer a zwitterionic character to the silaffin. Size, fine structure and yield of SiO2 spheres precipitated by silaffins depend on silaffins composition as well as the pH at which the reaction took place (for an overview on silaffins see Sumper and Kröger, 2004, J. Mater. Chem., 14, 2059-2065).
Our silaffin research focuses on structure-function-relationship between the design of peptidic polycations and their ability to precipitate SiO2 from a silicic acid solution. Both the conditions of precipitation as well as the qualification of the emerging strcutures are aspects that we aim to correlate with classified primary or secondary structures of short peptides.
As an initial sources for reference type polycations we chose different fragments of the unmodified silaffin sequence which were shown to be able to precipitate silicic acid. This allows us to manipulate primary structure both site specifically and with a random approach. We have set up an overexpression system in E.coli and the purified silaffin versions show specific precipitation of SiO2. Besides detailed analysis of assorted primary sequences we also follow an evolutive approach, scanning silica precipitation properties in a phage display system.
