| |
|
|
| |
|
|
Acidity constants and coupled proton/electron transfer.
|
Electron transfer is often coupled to proton transfer (PT) and this motivation has led us to develop a
first principle simulation approach to compute acidity constants.
The method is based on a half
reaction scheme computing free energies of dissociation from the vertical energy
gaps for insertion or removal of protons.
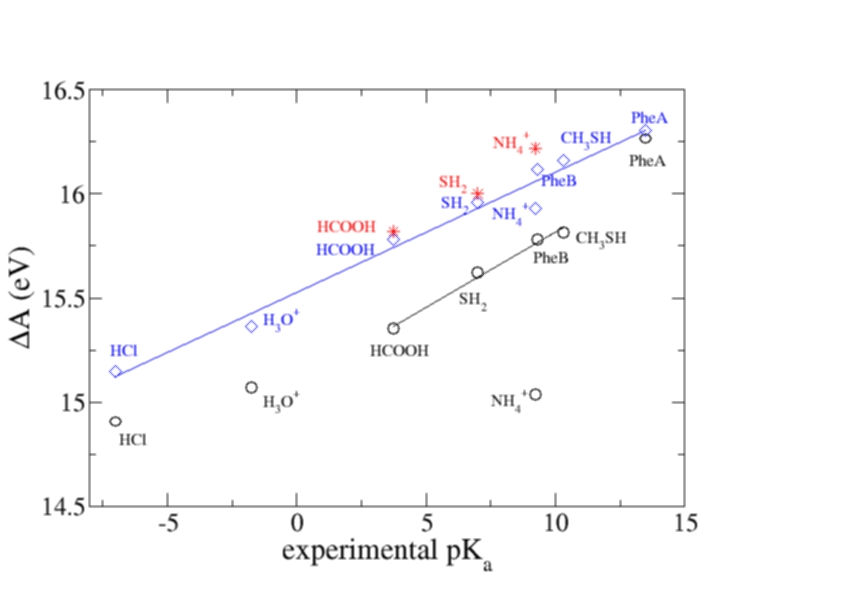
We verified
the method by investigating a series of organic and inorganic acids and
bases spanning
a wide range of pKa values (20 units) getting very promising results[3].
Our method is especially useful
in the cases where a continuum treatment of environment fails because of
the molecular structure of the solvent, or due to specific interactions
in the first
solvation shell. Recently we also used the moethod to calculate the
water dissociation
constant from first principles paving the way for a first
principles hydrogen electrode description.
The crucial point of the method is the consistent treatment of redox and
acid base reaction
enabling us to study proton coupled redox reactions.
We have extended this approach to the case of surface acidity (quartz-water interfaces and clay edges).
The
interaction of water with solid surfaces play a crucial role in many
phenomemna, for example the extend of protonation of
oxide surfaces has a fundamental inclfuence in the chemical behavior of
aqueous solution, controlling dissoluation/precipitation,
sorption, redox reactions. Accurate determination of solid surface
acidity is still subject of intense investigation due to the difficulty
to experimentally separate contribution coming from distinc functional
groups. | 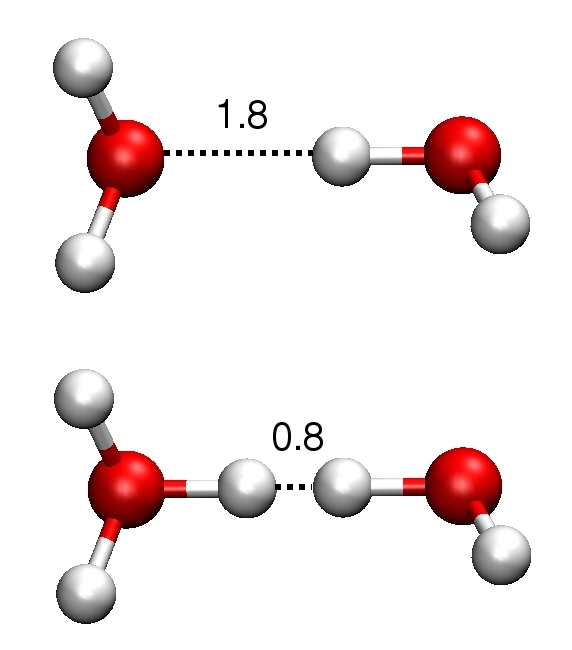
 |
M. Sulpizi, M. Sprik, Phys. Chem. Chem. Phys. 10 5238 (2008)
C. Adriaanse, M. Sulpizi, J. VandeVondele, M. Sprik, J. Am. Chem. Soc. 131 6046 (2009)
J. Cheng, M. Sulpizi, M. Sprik, J. Chem. Phys. 131 , 154504 (2009)
M. Sulpizi, M. Sprik, J. Phys.: Condens. Matter 22 , 284116 (2010)
F. Costanzo, R. G. Della Valle, M. Sulpizi, M. Sprik J. Chem. Phys. 134 , 244508 (2011)
M. Mangold, L. Rolland, F. Costanzo, M. Sprik, M. Sulpizi, J. Blumberger J. Chem. Theory Comp. 7 , 1951 (2011)
To contact me:
sulpizi@uni-mainz.de
Tel: +49 6131 3923641
Fax: +49 6131 3920496
Dr Marialore Sulpizi
Johannes Gutenberg University Mainz,
Staudinger Weg 7
55099 Mainz
Germany.