| |
|
|
| |
|
|
How the interface interactions influence the crystal growth.
Understanding the microscopic origin of gold nanoparticles anisotropic growth
|
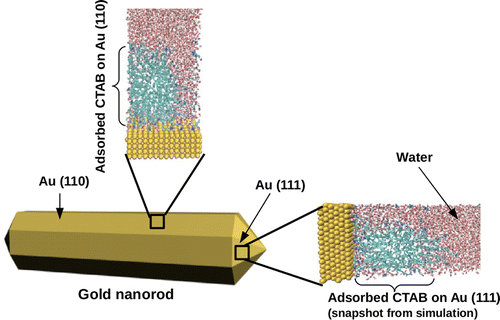
Gold nanorods are widely used in many areas such as drug delivery, photothermal cancer therapy, biochemical sensing and medical imaging. Growth of nanorods starts from small reasonably uniform gold seeds in a widely employed experimental technique, seed-mediated growth. In this technique ascorbic acid (a mild reducing agent), is added to aqueous solution of cetyltrimethylammonium bromide (CTAB) and AuCl4- (growth solution) for selective reduction of AuCl4- to AuCl2-, followed by the addition of the gold seed solution which catalyse the reduction of AuCl2- on their surface. Isotropic morphology breaking of the seeds by crystal twinning results into formation of pentatetrahedral structure with different facets and the selective interactions of CTAB to some specific facets of gold seed result into anisotropic growth and thus formation of nanorods. Although different growth mechanisms have been proposed, the microscopic understanding of anisotropic growth is still missing. |
|
We use molecular dynamics simulations in order to understand the microscopic origin of the asymmetric growth mechanism in gold nanorods. We provide the first atomistic model of the different surfaces on the gold nanoparticles in the growing electrolyte solution and we describe the interaction of the metal with the surfactants, namely the CTAB, and the ions. An innovative aspect is the inclusion of the role of the surfactants, which are explicitly modelled. We find that on all the investigated surfaces, namely (111), (110) and (100), the CTAB form a layer of distorted cylindrical micelles where channels among micelles would provide direct ions access to the surface. In particular, we show how AuCl2- ions, which are found in the growth solution, can freely diffuse from the bulk solution to the gold surface. We also find that the (111) surface exhibits the higher CTAB packing density and the higher electrostatic potential. Both elements would favour the grow of the gold nanoparticle along the (111) direction. These findings are in agreement with the growth mechanisms proposed by the experimental groups of Murphy and Mulvaney. S.K. Meena and M. Sulpizi Langmuir, 2013 DOI: 10.1021/la403843n. |
Calcium Oxalate/water interfaces and biomineralization (in collaboration with D. Donadio (MPIP Mainz)
|
Calcium oxalate is the most significant component of kidney stones. The presence of bio-polymers such as polyacrylate, poly-aspartate and poly-glutamate has a great impact on the crystalline phase, morphology and growth rate of i calcium oxalate. Ab initio molecular dynamics study of the interactions of the water/mineral and water/polymer/mineral interfaces shed light on the biomineralization process and on the mechanisms responsible for its inhibition. We performed DFT- based Molecular dynamics simulations to study the structure of the interfaces between calcium oxalate di-hydrate (COD) (100) and (101) and water. Our study reveals differences in the coordination of calcium ions at the surface with water, which is also responsible for different interactions with biopolymers. We also characterize the interaction between different surfaces of COD and biomolecules. As a first step we consider acetate as a model system containing a carboxylic group, and we estimate its binding structure and free energies on different COD surfaces at different coverage. Such free energies are then compared to the binding free energy of Ca and acetate in solution. Preferential binding of carboxylate to the 100 surface is found, therefore explaining recent experimental results on anisotropic growth of COD crystals in the presence of biopolymers. |
To contact me:
sulpizi@uni-mainz.de
Tel: +49 6131 3923641
Fax: +49 6131 3920496
Dr Marialore Sulpizi
Johannes Gutenberg University Mainz,
Staudinger Weg 7
55099 Mainz
Germany.