| |
|
|
| |
|
|
Solid/water interfaces structure and reactivity.
Environmentally relevant oxides-water interfaces
|
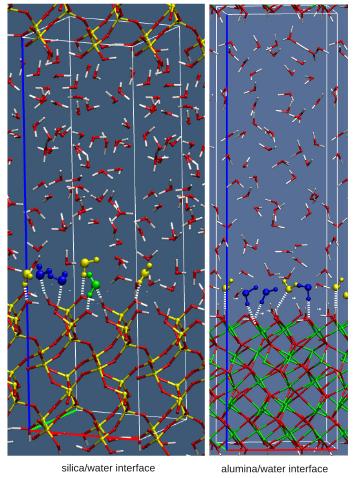
Silica is the most abundant metal oxide and the main component of the Earth's crust. Its behaviour in contact with water plays a critical role in a variety of geochemical and environmental processes. Despite its key role, the details of the aqueous silica interface at the microscopic molecular level are still elusive. We provided a detailed understanding of the molecular behaviour of the different oxide–water interfaces, using density functional theory based molecular dynamics (DFTMD) simulations, where a consistent treatment of the electronic structure of solvent and surface is provided. We also provided the first ab intio estimate of surface propensity to deprotonate using free energy perturbation methods. In particular for quartz we have calculated the acidity of the silanol groups and we find two types of silanol groups at the surface of quartz: out-of-plane silanols with a strong acidic character (pKa = 5.6), which consequently results in the formation of strong and short hydrogen bonds with water molecules at the interface, and in-plane silanols with a pKa of 8.5, forming weak hydrogen bonds with the interfacial water molecules. We have also shown how the silanols orientation and their hydrogen bond properties are responsible for an amphoteric behavior of the surface. A detailed analysis has identified two species of adsorbed water molecules at the solid–liquid interface, which using the language of vibrational spectroscopy can be identified as “liquid-like” and “ice-like” water or, in other words, water molecules forming respectively weak and strong H-bonds with the oxide surface. These two populations of water are in turn responsible for two distinct peaks in the infrared spectrum of interfacial water and thus provide a molecular explanation of the experimental sum frequency generation spectrum recorded in the literature. A similar analysis for alumina/water interface has shown that alumina oxides exhibits a quite different behaviour with respect to quartz. In particular the way the aluminol groups influence water structure at the interface has been discussed and compared to the experiments. |
|
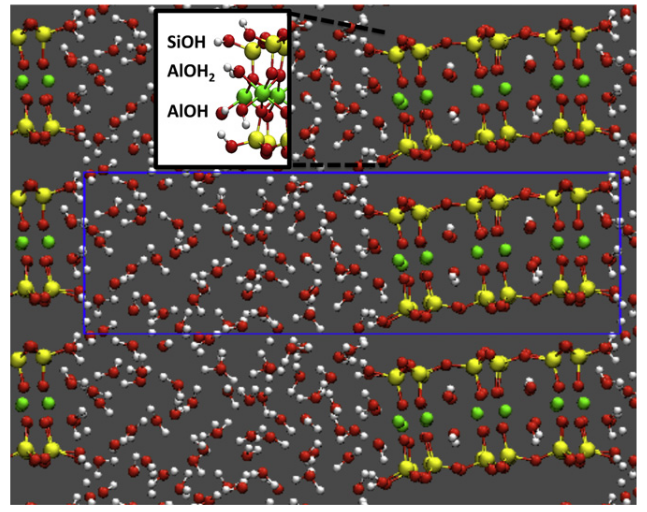
We provide a microscopic understanding of the solvation structure and reactivity of the edges of neutral clays. In particular we address the tendency to deprotonation of the different reactive groups on the (010) face of pyrophyllite. Such information cannot be inferred directly from titration experiments, which do not discriminate between different sites and whose interpretation resorts to macroscopic models. The determination of the corresponding pKa then usually relies on bond valence models, sometimes improved by incorporating some structural information from ab-initio simulations. We find that the most acidic group is SiOH, due to its ability to establish strong hydrogen bonds with adsorbed water, as it also happens on the quartz and amorphous silica surfaces. The acidity constant of AlOH2 is only 1 pKa unit larger. Finally, the pKa of AlOH is outside the possible range in water and this site should not deprotonate in aqueous solution. We show that the solvation of surface sites and hence their acidity is strongly affected by the proximity of other sites, in particular for AlOH and AlOH2 which share the same Al. We discuss the implications of our findings on the applicability of bond valence models to predict the acidity of edge sites of clays. |
The silica/water interface: how the silanols determine the surface acidity and modulate the water properties M. Sulpizi, M.-P. Gaigeot, M. Sprik J. Chem. Theory Comput., 2012, 8 (3),1037 (2012)
Oxide/water interfaces: how the surface chemistry modifies interfacial water properties M.-P. Gaigeot, M. Sprik, M. Sulpizi J. Phys. Cond. Matt. 24(12), 124106 (2012)
The Amorphous Silica Water Interface Studied by Ab Initio Molecular Dynamics (AIMD): Local Organization in a Global Disorder. A. Cimas, F. Tielens, M. Sulpizi, M-P. Gaigeot and D. Costa (2013) submitted to J. Phys.: Cond. Matt.
Absolute acidity of clay edge sites from ab-initio simulations S. Tazi, B. Rotenberg, M. Salanne, M. Sprik, and M. Sulpizi Geochimica et Cosmochimica Acta, 94, 1 (2012)
To contact me:
sulpizi@uni-mainz.de
Tel: +49 6131 3923641
Fax: +49 6131 3920496
Dr Marialore Sulpizi
Johannes Gutenberg University Mainz,
Staudinger Weg 7
55099 Mainz
Germany.